CPSC Issues Airborne Beach Umbrella Warning
 The Consumer Product Safety Commission has issued a warning that airborne beach umbrellas can kill explaining that incidents with these umbrellas have become “all too common.” Wind gusts have pulled up beach umbrellas on crowded beaches and sent them hurtling towards people. The results have been deaths and serious injuries like lacerations and impalements. There is now a voluntary safety standard to assist manufacturers in making sure that the danger is minimized. If your loved one was killed or you were injured by a beach umbrella that went flying, you may be able to recover compensation. The seasoned Chicago-based product liability lawyers of Moll Law Group can go over your legal options.
The Consumer Product Safety Commission has issued a warning that airborne beach umbrellas can kill explaining that incidents with these umbrellas have become “all too common.” Wind gusts have pulled up beach umbrellas on crowded beaches and sent them hurtling towards people. The results have been deaths and serious injuries like lacerations and impalements. There is now a voluntary safety standard to assist manufacturers in making sure that the danger is minimized. If your loved one was killed or you were injured by a beach umbrella that went flying, you may be able to recover compensation. The seasoned Chicago-based product liability lawyers of Moll Law Group can go over your legal options.
Call Moll Law Group About Your Beach Umbrella Claim
The new voluntary safety standard ASTM F3681-24 requires that beach umbrellas and their anchor systems, along with beach umbrellas that are used with complaint anchors need to offer a resisting force of at least 75 pounds. Alternatively, they need to stay secure in wind speeds of up to 30 miles per hour, when the compliant anchor is secured in the sand.
Its expected that products compliant with this safety standard will be available soon. Consumers should look at the labels of anchors, which will state whether it meets the new safety standard by indicating that it “MEETS ASTM F3681 FOR WIND SPEEDS UP TO 30 MPH.” This will also set forth the maximum canopy size for an umbrella used with the anchor.
 Illinois Injury and Mass Tort Lawyer Blog
Illinois Injury and Mass Tort Lawyer Blog


 On May 30, 2024, the manufacturer Black & Decker
On May 30, 2024, the manufacturer Black & Decker  Recently around 1.6 million Black+Decker garment steamers were
Recently around 1.6 million Black+Decker garment steamers were 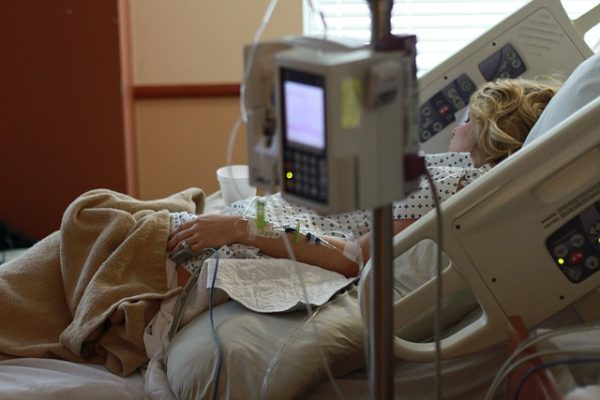 Around 15 million Philips Respironics CPAP and ventilator machines were impacted by a Class 1 recall. This class of recall is the most serious because it indicates that it’s reasonably probable that using or just being exposed to the affected products will result in serious adverse health ramifications or even deaths. Recently, the manufacturer Philips reached a
Around 15 million Philips Respironics CPAP and ventilator machines were impacted by a Class 1 recall. This class of recall is the most serious because it indicates that it’s reasonably probable that using or just being exposed to the affected products will result in serious adverse health ramifications or even deaths. Recently, the manufacturer Philips reached a  Doctors sometimes perform total hip arthroplasty or total hip replacement using the Synovo Total Hip System, which features resurfacing implants. The United States Food and Drug Administration (FDA) has
Doctors sometimes perform total hip arthroplasty or total hip replacement using the Synovo Total Hip System, which features resurfacing implants. The United States Food and Drug Administration (FDA) has 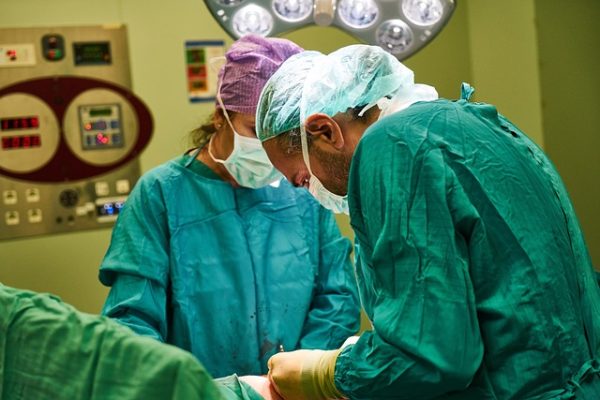 Recently, Asensus Senhance Surgical System
Recently, Asensus Senhance Surgical System  Recently, Walmart, Costco, and Trader Joe’s recalled certain cheeses after a
Recently, Walmart, Costco, and Trader Joe’s recalled certain cheeses after a  In November 2023, a 6 month baby died in a Fisher-Price infant-to-toddler rocker.
In November 2023, a 6 month baby died in a Fisher-Price infant-to-toddler rocker.  Due to severe health risks, the Food and Drug Administration (FDA) has warned against buying or using tianeptine products like Neptune’s Fix. Tianeptine supplements are commonly sold at convenience stores and gas stations under the name Neptune’s Fix. The drug tianeptine has not been approved for medical use in this country, though it is prescribed as an antidepressant in other countries. Synthetic pot was also found in supplements. On January 29, 2024, Neptune’s Fix was
Due to severe health risks, the Food and Drug Administration (FDA) has warned against buying or using tianeptine products like Neptune’s Fix. Tianeptine supplements are commonly sold at convenience stores and gas stations under the name Neptune’s Fix. The drug tianeptine has not been approved for medical use in this country, though it is prescribed as an antidepressant in other countries. Synthetic pot was also found in supplements. On January 29, 2024, Neptune’s Fix was  Recently, a jury ordered Bayer AG’s Monsanto unit to pay more than
Recently, a jury ordered Bayer AG’s Monsanto unit to pay more than